As a pharmacist, Kathy James considers herself well educated about the importance of getting regular cancer screenings. Even though the 55-year-old had no history of cancer in her family, she never skipped her regular mammograms, and she gave herself regular breast exams. So she was dumbfounded when, during one of those self-exams in May 2017, she felt a marble-size lump in her left breast. A visit to the doctor confirmed it. “The radiologist came in with his hands in his pockets and looked down and said, ‘It doesn’t look good,'” James says. After a biopsy, James and her husband learned she had metastatic breast cancer. It was their 26th wedding anniversary.
James immediately wanted to have both breasts surgically removed, which she believed would drastically reduce the chance that the cancer would spread. “I wanted to be done with it all,” she says. “I was hell-bent on getting the double mastectomy.”
If James had been diagnosed even five years earlier, she likely would have gone through with the radical surgery, even though it would not have guaranteed her cancer would not spread, or return. A friend who is a cancer doctor advised her against getting the surgery and referred her to Dr. Brian Czerniecki at Moffitt Cancer Center, who told her about a pioneering study he was conducting to test a completely new way of fighting breast cancer–not solely with chemotherapy, radiation or surgery but by harnessing the power of her own immune system. The study and dozens of others like it are potentially rewriting the manual for breast-cancer care, giving patients unprecedented options for controlling their disease and possibly even curing it.
The immune system mounts the body’s defense and offense against unwanted intrusions: bacteria, viruses and even cancer cells. Cancer, however, poses a tricky problem. Malignant cells develop from normal cells that start to grow out of control, and the immune system is specifically programmed not to attack the body’s own cells.
But scientists have found ways to retrain the body to recognize and destroy tumor cells, making immune-based treatments the newest, most promising weapon against many types of cancer. The first of these immunotherapy drugs approved to treat cancer began in the labs of James Allison of MD Anderson Cancer Center and Dr. Tasuku Honjo of Kyoto University in the 1990s. They independently discovered different ways in which the immune system is blocked from attacking tumor cells, which just earned them the 2018 Nobel Prize in Physiology or Medicine. Their finding led to a new class of drugs, called checkpoint inhibitors, that allow the immune system to see cancer cells as the disease-causing rogues they are and attack them, drastically improving remission rates. In the past five years, the Food and Drug Administration (FDA) has approved a dozen new cancer drugs and therapies that exploit the immune system. “All cancer patients will likely receive [immunotherapy] in five years, so it’s going to be curative for a lot of them,” Allison says.
Immunotherapy treatments are especially effective against lung cancer, skin cancer and blood cancers like leukemia and lymphoma. But immune-based treatments have not been as successful, or as plentiful, for the most common cancers: colon, prostate and especially breast. Of the more than 600,000 people who died of cancer in the U.S. in the past year, the vast majority had these types of solid tumors. The problem, says Dr. Robert Vonderheide, director of the Abramson Cancer Center at the University of Pennsylvania, is that “most breast cancers fit into a category we call ‘cold’ immunological tumors, meaning the tumor has the ability to either exclude the immune system or hide from it altogether. That kind of cancer isn’t easily treated with current immunotherapies.”

At least not yet. Building on the foundation that Allison and Honjo established with checkpoint inhibitors, researchers are finding creative ways to stimulate the immune system to see tumors like breast cancer as “hot” rather than “cold” targets, in the same way infectious-disease bugs like measles or influenza viruses flag a response. They are accomplishing this by learning from successful treatments for diseases like tuberculosis and HIV, which combine therapies; against cancer, they match immune-based treatments with more traditional ones like chemotherapy, surgery or radiation to boost whatever immune response there is. While chemotherapy and radiation in their current forms destroy immune cells along with malignant ones, modified formulas may be just enough to stimulate an inflammatory response that awakens the immune system to see tumor cells. Breast-cancer researchers are testing ways to then deploy immunotherapy drugs like checkpoint inhibitors to elicit the strongest immune response against tumors.
James was intrigued by the idea of training her body to fight her cancer as a more sustainable solution than short-term cycles of chemotherapy or radiation alone. “Because my cancer tends to recur, and because it’s aggressive, I couldn’t just stop once my year of treatment was up,” she says. “I needed to keep going.” And now, for patients like her, more creative ways to prime the immune system against cancer are starting to emerge. At the National Cancer Institute, Dr. Steven Rosenberg, chief of surgery, studies the mutations that drive his patients’ breast cancer, isolates the few immune cells that are trying to fight the cancerous cells, amplifies their numbers in the lab and infuses them back into his patients. He’s convinced that the strategy could become the blueprint for translating the same success that immunotherapies have had in blood, lung and skin cancers to the more common malignancies in the breast, prostate and colon.
There’s a reason doctors focused their first immunotherapy efforts on cancers like melanoma and lung. These diseases tend to emerge because cells build up many mutations, or mistakes in their DNA, that instruct them to start growing abnormally and out of control. Normally, mutations make a cancer difficult to treat, because they allow the cancer to form new ways to dodge treatments launched against it. But when it comes to making immunotherapies work, they’re a definite plus. In the 1980s, Rosenberg became among the first to notice that those mutations also attract the attention of the immune system, and some immune cells can start infiltrating the tumors.
At the time, Rosenberg says, “nobody knew there was an immune response against human cancers.” He decided to try to use it to his advantage and eventually developed a way to isolate these cancer-fighting immune cells, known as T cells, from 195 people with melanoma, expanding their numbers and infusing them back to the patients. So far, 30% of them have responded completely to the therapy, meaning their existing cancer cells disappeared and they have not seen any tumors reappear in nearly seven years since the treatment.
Encouraged by that show of immunological force, doctors began exploring ways to exploit the immune system to treat other cancers. Leukemias and lymphomas, which form when blood cells turn malignant, can’t be treated with surgery and tend to recur even after chemotherapy and radiation. But they are particularly amenable to a type of immune therapy that involves replacing a patient’s malignant blood with a population of their T cells that are processed to attack a receptor common among cancerous blood cells. Up to 90% of people with certain types of leukemia whose cancer recurred after repeated cycles of standard treatments have gone into remission after receiving this form of immunotherapy. Such success led the FDA to approve the first immune-cell-based therapy, called CAR T, for a type of leukemia and other blood cancers in 2017.
But solid cancers–which are much more common–have fewer mutations, and the tissues they invade (like those in the breast) can’t be replaced as blood cells can, making immunotherapy more difficult. Vonderheide has found that of the 7,000 tumors listed in a national genetic database, breast cancers fell in the bottom 25% of tumors when it came to how many mutations they carried. Because of that, breast cancers are also part of the bottom half of all cancers when it comes to immune responses the body generates against them. “Breast cancer notoriously shields itself from the immune system,” he says.
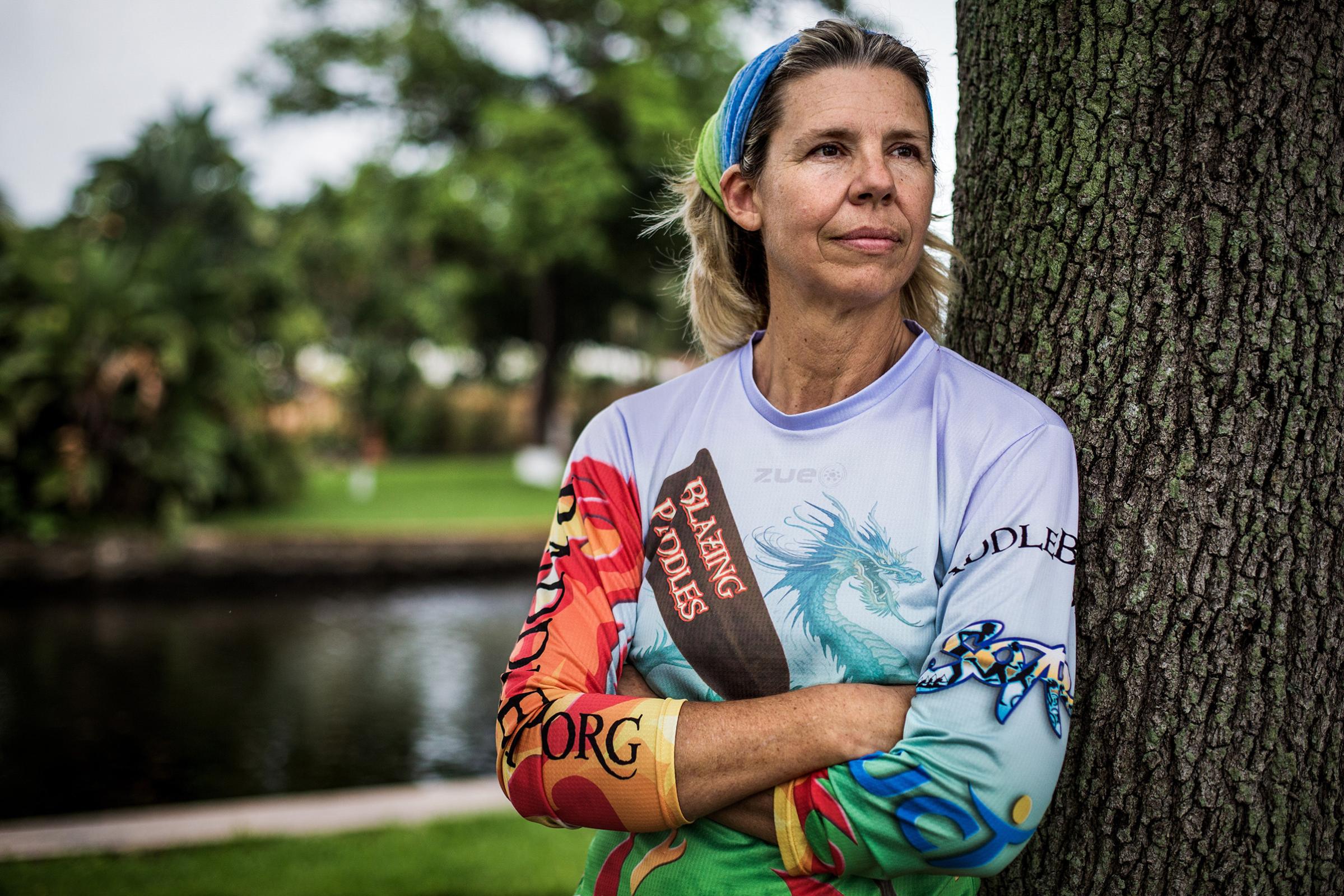
Now Rosenberg is exploring ways to unlock breast cancer to the possibilities of immunotherapy. Building on his early work with T cell responses to cancer, he designed an experimental therapy customized to each patient’s cancer and tested it first in people with liver, colon and cervical cancer. The first breast-cancer patient in his study, Judy Perkins, had Stage IV cancer that had recurred and spread to lumps in her chest and to her liver, despite a dozen chemotherapy and hormone treatments and even a mastectomy. Tapping into the growing knowledge of how genes drive cancer, Rosenberg did a thorough genetic analysis of her tumor and found 62 major mutations responsible for turning Perkins’ cells malignant. He then searched for the valiant few immune cells that could recognize and attack four of those genetic aberrations and were already battling her cancer. He extracted those immune cells, grew them in larger numbers in the lab and returned them to Perkins via IV as an immune-based treatment against her breast cancer.
Having exhausted all of her treatment options, Perkins had said her goodbyes to loved ones and was “waiting for the end,” she says. But within a month of receiving the onetime infusion of cells, she felt the tumor in her chest get “softer and smaller.” Within two months, the tennis-ball-size growth in her liver had disappeared and the tumor in her chest had also shriveled to nothing. Nearly three years later, doctors say she is in a durable regression. “I am totally thrilled. It’s awesome,” she says. But Perkins knows for now she’s an exception. So far, only 14% of the 42 people Rosenberg has treated have responded as Perkins has. Rosenberg believes that percentage will increase if he and others find better ways to pinpoint both the mutations behind each patient’s cancer and the population of immune cells targeted against them. As that science evolves, it could bring immunotherapy to not just those with breast cancer but those with other solid tumors as well. “This could basically be a blueprint for the treatment of any cancer type,” he says. “And I frankly think that it’s got a good chance of working.”
There are limits. Rosenberg’s method of immunotherapy is a time-consuming and expensive treatment, and since it requires a customized approach, it can’t be mass-produced as a universal, off-the shelf procedure for any patient. There need to be other ways to tap into an immune response–which is why Czerniecki is testing possible “vaccines” in James and other patients that would search for and destroy cancer cells before they recur as tumors.
James’ breast cancer is HER2-positive, named for the protein that dominates her cancer cells–a protein that initially attracts an immune response but then loses it over time. Czerniecki created a vaccine that stimulates that response again. “We’re trying to restore some of the immune responses that get knocked out or dampened over time,” he says.
It’s helpful, but not enough in James’ case. As the disease progresses, cancer cells actively shield themselves from the immune system by blanketing themselves in proteins that are found in abundance on neighboring healthy cells, so targeting the cancer cells can also kill normal ones. “With invasive tumors, we have to play with their environment, because it has become almost like a wall to the immune system that we’ve got to penetrate,” Czerniecki says.
One way to accomplish that, ironically, is to enlist the help of older methods of treating breast cancer. At Penn, Vonderheide is experimenting with ways to combine newer immunotherapy drugs with conventional treatments like chemotherapy and radiation with the hope that the synergistic effect will make tumors more visible and vulnerable to immune attack. The idea, Nobel Prize winner Allison says, is to “turn radiation and chemo into a sort of vaccine.”
The key is not using standard cycles of chemo or radiation, but tweaking the treatments so they are just right for activating an immune response. Too much chemo or radiation suppresses the immune system, but just enough can act like a stimulant to activate it. “It’s almost as if chemotherapy can roughen up the surface of cancer cells and get the [proteins] released that attract the immune system,” says Dr. Peter Schmid, clinical director of the breast-cancer center at the St. Bartholomew Cancer Centre in London.
Schmid will announce much anticipated results at the end of October from a study that combines a chemotherapy agent combined with a checkpoint inhibitor for treating advanced triple-negative breast cancer, an aggressive, difficult-to-treat form of the disease. The chemo is delivered in nanoparticle form, which makes it more soluble and better equipped to slip inside cell membranes to activate an immune response. “Patients ask me every day, ‘Why do I need chemotherapy? Won’t it bring my immune system down?'” Schmid says. “We are only just understanding that it can have a more subtle and positive effect on immunity against cancer.”
There is similar excitement over combining shorter schedules of radiation with checkpoint inhibitors. This approach shows even more promise as a way to target tumors that have spread to hard-to-reach tissues–a common issue with breast cancer. Researchers believe that’s because radiation given over a few days rather than over the standard weeks-long schedule may be enough to trigger an immune response against a specific tumor, which is then directed to attack tumor cells in other parts of the body. In the case of breast cancer, researchers hope that this response will find growths that have spread beyond the breast and target those. This new thinking, Allison says, is “really disruptive, because we’re realizing with chemo and radiation that we don’t need to kill every last tumor cell, but stir up things just enough for the immune system to take them out.”
Studies like the one in which James is participating could also overturn the current thinking about how to treat one of the stubborn challenges of breast cancer–recurring tumors. If vaccines designed to awaken immune cells against cancer are effective, then breast-cancer patients could potentially be protected from having their cancer return with periodic anticancer “booster” shots. Their immune systems would essentially be primed to find and eliminate any cancer cells before they can coalesce into tumors.
James and Perkins are hopeful that their participation will speed immune-based treatments into regular rotation for future breast-cancer patients. James received six inoculations of her cancer-fighting immune cells over the summer and is scheduled to receive three boosters beginning in January. “I can’t change the fact that I have breast cancer, but I can pay it forward by being part of a clinical trial in the hopes that there will be a vaccine my children and grandchildren will get, so they wouldn’t have to endure what I went through,” she says. Perkins just wants her miraculous case to become the routine rather than the exception. “The immune system has such potential, and we are just starting to crack this door open,” she says. “I’m hoping that door will open all the way and we have more effective treatments. I would like some company in being the golden guinea pig.”
More Must-Reads From TIME
- Dua Lipa Manifested All of This
- Exclusive: Google Workers Revolt Over $1.2 Billion Contract With Israel
- Stop Looking for Your Forever Home
- The Sympathizer Counters 50 Years of Hollywood Vietnam War Narratives
- The Bliss of Seeing the Eclipse From Cleveland
- Hormonal Birth Control Doesn’t Deserve Its Bad Reputation
- The Best TV Shows to Watch on Peacock
- Want Weekly Recs on What to Watch, Read, and More? Sign Up for Worth Your Time
Contact us at letters@time.com